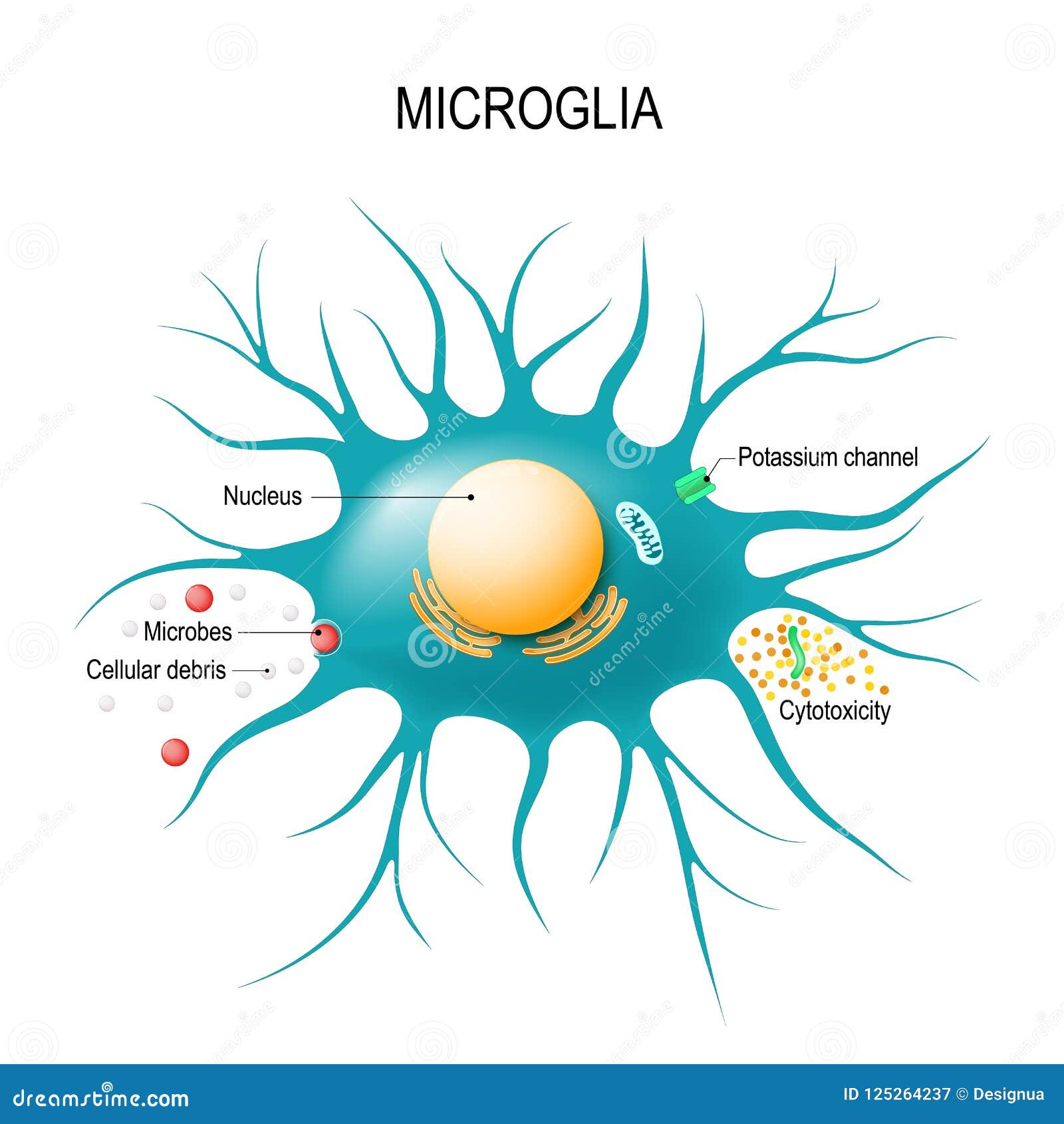
Microglial cells are a fascinating yet underappreciated component of the brain’s immune system, playing a crucial role in maintaining the delicate balance within our neurological environment. Recent advances in Alzheimer’s research have illuminated how these cells actively patrol the brain, seeking out signs of damage and disease while also participating in the pruning of synapses to facilitate effective communication among neurons. However, the role of microglia is a double-edged sword, as aberrant functioning of these cells can lead to the progression of neurodegenerative diseases, such as Alzheimer’s and Huntington’s. Pioneering research from scientists like Beth Stevens has unveiled groundbreaking insights into microglia, fostering a deeper understanding of their complex interactions, which are pivotal for both healthy brain development and disease emergence. With ongoing neuroscience breakthroughs, we are now better equipped to explore potential therapeutics that could alter the course of these devastating conditions, providing hope for millions affected by Alzheimer’s.
Often referred to as the brain’s resident immune agents, microglia are specialized cells that fulfill vital protective and regulatory functions within the central nervous system. These crucial components help regulate inflammation and maintain homeostasis in the brain, but their malfunction can lead to significant problems, particularly in the context of age-related cognitive decline. As researchers delve deeper into neuroinflammatory pathways, especially those discovered by experts like Beth Stevens, the understanding of how these immune cells interact with neural networks continues to evolve. This line of inquiry not only enhances our knowledge of various neurological disorders but also advances our ability to develop novel interventions for conditions like Alzheimer’s. Emphasizing the critical nature of this research is essential for grasping the broader implications of neurological health and disease management.
The Role of Microglial Cells in Alzheimer’s Research
Microglial cells are crucial components of the brain’s immune system, acting as sentinels that detect and respond to injury or disease. Recent research led by Beth Stevens has illuminated the significant role these cells play in maintaining neural health and function. Not only do microglial cells assist in the clearance of dead cells, but they also engage in the pruning of synapses, which is essential for optimal neuronal communication. However, dysregulation in the activity of microglia can lead to excessive pruning, a process linked to neurodegenerative diseases such as Alzheimer’s and Huntington’s. Understanding this balance is vital in devising effective strategies against such debilitating conditions, ultimately aiming to improve the lives of the millions affected by these diseases.
Stevens’ groundbreaking work has paved the way for investigating how microglial dysfunction contributes to Alzheimer’s pathology. Through careful analysis, her lab has shown that abnormal microglial pruning is associated with synaptic loss and cognitive decline. This discovery not only advances our understanding of the mechanisms underlying Alzheimer’s disease but also opens new avenues for developing biomarkers that could lead to early diagnosis and treatment interventions. As we delve deeper into the intricacies of microglial function, it becomes clear that these immune cells hold the key to unlocking potential therapeutic targets that could alter the course of neurodegenerative diseases.
Understanding the Brain’s Immune System: Insights from Beth Stevens
Beth Stevens has been at the forefront of neuroscience breakthroughs that highlight the importance of the brain’s immune system, particularly concerning disorders like Alzheimer’s disease. Her research emphasizes that microglial cells are not merely defenders against pathogens; they are integral players in synaptic development and plasticity. Essentially, these cells shape the neural circuitry that underpins learning and memory. This pivotal insight has transformed how researchers view brain health and its connection to a myriad of neurodegenerative diseases, reinforcing the notion that the immune system’s role extends beyond traditional boundaries.
Furthermore, Stevens’ emphasis on curiosity-driven science underscores the unpredictable nature of research, especially in a field as complex as neuroscience. Her journey illustrates the necessity of foundational research that seems distant from clinical application but ultimately holds the potential for groundbreaking discoveries. By investigating the workings of microglia in various contexts, Stevens has contributed to a deeper comprehension of how disruptions in the immune response can lead to neurodegeneration. This not only enriches our understanding of Alzheimer’s pathology but also fuels the quest for innovative therapeutic strategies.
Neuroscience Breakthroughs and Their Impact on Neurodegenerative Diseases
The landscape of neuroscience is rapidly evolving, with breakthroughs providing new insights into the mechanisms of neurodegenerative diseases. This evolution is particularly crucial for conditions like Alzheimer’s disease, where understanding cellular interactions can unveil new treatment possibilities. Researchers are now aware that microglial cells have both protective and harmful roles in the brain. By establishing more effective methods to leverage their protective capabilities while curtailing their detrimental effects, scientists can formulate innovative strategies aimed at combating these debilitating diseases.
Moreover, Stevens and her colleagues demonstrate that breakthroughs in understanding the pathophysiology of neurodegenerative diseases can stem from basic scientific inquiries. The research trajectory often begins with fundamental questions about brain function, such as how synapses are formed and which cells are involved in maintaining brain integrity. As these inquiries deepen, they reveal connections to neurodegenerative conditions, fueling a cycle of discovery that can lead to transformative treatments. This highlights the critical importance of investing in basic research to foster progress across the medical landscape.
Alzheimer’s Disease: The Need for Innovative Treatments
The urgency for innovative treatments for Alzheimer’s disease has never been more critical, given the staggering number of individuals affected by it. With over 7 million Americans currently living with this ailment, researchers like Beth Stevens are focused on unlocking the mysteries of the brain’s immune system to identify potential therapies. Understanding how microglial cells behave in the context of Alzheimer’s offers hope that new intervention strategies could be developed. Targeting the pathways involved in abnormal microglial activity could lead to breakthroughs in treating or even preventing this debilitating disease.
Stevens’ work emphasizes that the fight against Alzheimer’s requires a multifaceted approach that integrates our growing knowledge of neurobiology and immune response. Current treatments aim to alleviate symptoms, but the ultimate goal is to halt or reverse the progression of the disease. With innovative research exploring the role of the brain’s immune system and its interplay with neurodegenerative processes, we are inching closer to finding a cure. The commitment to understanding and harnessing the potential of microglial cells could lead to transformative advancements in the care and treatment of Alzheimer’s disease.
The Importance of Federal Funding in Neuroscience Research
Federal funding has been a cornerstone in propelling neuroscience research forward, particularly in understanding complex diseases like Alzheimer’s. Researchers such as Beth Stevens have benefited immensely from support provided by institutions like the National Institutes of Health (NIH). This robust backing has allowed for detailed exploration into the roles of microglial cells and their implications for neurological health. Without sufficient funding, many groundbreaking studies that seek to unveil the underpinnings of neurodegenerative diseases may never take place, stymying innovation in treatment and care.
Moreover, the commitment to funding basic science acts as a catalyst for curiosity-driven discoveries that can pave the way for significant clinical application. Stevens articulates the unpredictable yet rewarding nature of research, where seemingly unrelated findings can converge to address pressing health issues. Federal investment fuels the possibility of exploring innovative questions that deepen our understanding of brain function and pathology, leading to scientific advancements that could ultimately result in effective therapies for Alzheimer’s disease and other neurodegenerative disorders.
The Link Between Microglial Dysfunction and Synaptic Pruning
Research has increasingly shown that microglial dysfunction is intricately linked to aberrant synaptic pruning in neurodegenerative diseases. Studies from the Stevens Lab have illustrated that in conditions like Alzheimer’s, microglia may prune excessively or inappropriately, leading to synaptic loss and cognitive impairment. This is a key area of focus in understanding how Alzheimer’s develops and progresses, making it essential to investigate the dynamics of microglial behavior further. The implications of these findings underscore the possibility of restoring normal microglial function as a novel therapeutic avenue.
Understanding the mechanisms that regulate microglial pruning can illuminate pathways that, when disrupted, lead to neurodegenerative changes. Researchers are now exploring how to harness the potential of microglial cells to promote healthy synaptic pruning while preventing the pathological consequences of their dysregulation. The goal is to develop strategies that enhance the brain’s resilience against diseases like Alzheimer’s, ultimately protecting cognitive abilities and improving quality of life for individuals suffering from these conditions.
Exploring Biomarkers for Early Alzheimer’s Diagnosis
The quest for reliable biomarkers for early detection of Alzheimer’s disease is more crucial than ever, as research continues to signify the importance of microglial cells. Beth Stevens’ work provides insights into how changes in microglial activity could serve as indicators of the onset of Alzheimer’s. Identifying specific markers that correlate with abnormal microglial behavior could revolutionize how we diagnose and monitor the disease, leading to earlier interventions and potentially more effective treatment options.
By integrating knowledge of microglial function and its relation to neuropathology, researchers can develop comprehensive biomarker panels that reflect the complex interactions within the brain. These biomarkers would not only aid in identifying Alzheimer’s in its early stages but can also provide information about disease progression, enhancing the ability to tailor treatments to individual patients’ needs. The development of precise, microglial-based biomarkers represents a promising step toward more personalized approaches in the fight against neurodegenerative diseases.
The Future of Alzheimer’s Research: Collaborative Endeavors
As the field of Alzheimer’s research evolves, collaboration among scientists, clinicians, and stakeholders has become increasingly vital. The interconnected nature of neurobiology, immunology, and clinical application necessitates a multidisciplinary approach to tackle the complexities of Alzheimer’s disease. Beth Stevens exemplifies this collaborative spirit, working with various institutions to propel research forward and foster innovations in treatment and care. Through teamwork and shared knowledge, the scientific community is better equipped to unveil new pathways and strategies to combat neurodegeneration.
Moreover, collaborative efforts are essential for pooling resources and expertise to tackle challenging questions related to Alzheimer’s disease. By bringing together diverse perspectives and insights, researchers can accelerate progress in understanding the intricacies of microglial function and its impact on neurodegenerative disorders. This collective advancement not only enhances the body’s knowledge base but also fosters a more supportive environment for developing cutting-edge therapies that could transform the lives of patients affected by Alzheimer’s and other neurodegenerative diseases.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s research?
Microglial cells are crucial in Alzheimer’s research as they function as the brain’s immune system. They help clear dead or damaged cells and prune synapses, which is important for healthy brain function. However, in Alzheimer’s disease, abnormal microglia activity can lead to detrimental outcomes like excessive synaptic pruning, contributing to neurodegeneration.
How do microglial cells interact with neurodegenerative diseases?
Microglial cells interact with neurodegenerative diseases such as Alzheimer’s and Huntington’s disease by mediating immune responses in the brain. They are activated in response to neuronal injury and play a role in the clearance of toxic debris, but dysregulation of their function can exacerbate disease progression, highlighting their dual role in both protecting and harming brain health.
What neuroscience breakthroughs have been made regarding microglial cells?
Recent neuroscience breakthroughs regarding microglial cells include understanding their essential role in synaptic pruning and how they contribute to neuroinflammation in diseases like Alzheimer’s. Research led by scientists like Beth Stevens has illuminated pathways through which microglia can become dysfunctional, paving the way for potential new therapies to target these processes in neurodegenerative diseases.
How do microglial cells contribute to the brain immune system?
Microglial cells are integral to the brain’s immune system, serving as its primary immune surveillance and response cells. They constantly monitor the brain environment for signs of disease or injury, and when activated, they can phagocytose (ingest) debris and dead cells, thus helping to maintain homeostasis and supporting neuronal health.
What implications do microglial studies have for Alzheimer’s treatment?
Studies of microglial cells have significant implications for Alzheimer’s treatment. By understanding how these cells malfunction in diseases, researchers can identify new biomarkers and therapeutic targets to potentially halt or reverse neurodegenerative processes associated with Alzheimer’s, aiming to improve the quality of life for those affected.
Who is Beth Stevens and what is her contribution to microglial research?
Beth Stevens is a neuroscientist who has made groundbreaking contributions to understanding microglial cells and their role in the brain’s immune system. Her research has revealed how dysfunctional microglial activity can contribute to neurodegenerative diseases like Alzheimer’s, establishing a foundation for new biomarkers and treatment strategies aimed at these conditions.
What is the impact of microglial cells on neuroinflammation?
Microglial cells are key regulators of neuroinflammation. They respond to neuronal injury and other stimuli by becoming activated and releasing inflammatory cytokines. While they play a protective role, chronic activation can lead to excessive inflammation, contributing to the progression of neurodegenerative diseases such as Alzheimer’s and impacting overall brain health.
What advancements have been made in understanding microglia’s role in synaptic pruning?
Recent advancements in understanding microglia’s role in synaptic pruning have shown that these cells are not just immune sentinels; they are actively involved in removing excess synapses during brain development and in response to injury. Dysregulated pruning by microglia has been implicated in neurodegenerative diseases like Alzheimer’s, highlighting the importance of careful regulation of their activity.
| Key Points | Details |
|---|---|
| Microglial Cells | Act as the brain’s immune system, patrolling for illness or injury. |
| Pruning Process | Help clear dead or damaged cells and selectively prune synapses that transmit information among neurons. |
| Impact of Abnormal Pruning | Can contribute to neurodegenerative diseases such as Alzheimer’s and Huntington’s disease. |
| Research Contributions | Foundation for new biomarkers and treatments for Alzheimer’s and other neurodegenerative diseases established by Stevens’ research. |
| Funding Sources | Significant support from NIH and other federal agencies facilitated research advancements. |
| Broader Implications | Basic science can lead to unexpected discoveries that improve understanding and treatment of diseases. |
Summary
Microglial cells play a critical role in maintaining brain health and are essential in the fight against neurodegenerative diseases such as Alzheimer’s. Understanding their function not only aids in comprehending the brain’s immune system but also paves the way for innovative treatments. Continued research led by scientists like Beth Stevens is crucial, as it opens avenues for developing new biomarkers and therapeutic options that can significantly improve the lives of millions suffering from conditions like Alzheimer’s.